Research Suggests Bile Acids Have Potential as a Therapy for Dysbiosis, Constipation, and Inflammatory Bowel Disease
Generally, when we think of bile, we first think of the role it plays in digestion. Produced by the liver and expelled into the digestive tract by the gallbladder, bile is the substance that serves to emulsify and break down dietary fats so that they can be absorbed in the small intestine. Thus, supplemental bile acids with meals may be important for individuals post-cholecystectomy or with fat malabsorption for other reasons. However, the effects and potential therapeutic benefits of bile acids in the body go far beyond this.
In the digestive tract, bile acids also affect the balance of flora and gut motility.[1],[2] Outside of the gut, they regulate many critical facets of physiology, including glucose and cholesterol metabolism; activating farnesoid X receptor (FXR), pregnane X receptor, the vitamin D receptor, and various G-protein-coupled receptors.[5] Evidence also suggests that bile acids affect neurological function, as well as the response of the hypothalamic–pituitary–adrenal axis.[6] Bile acids have even been suggested to be “novel therapeutic modalities in inflammation, obesity, and diabetes.”[7]
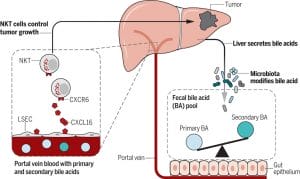
Although collectively referred to in the general practice of medicine as bile or the bile salt pool, the functions of bile acids and their metabolites vary considerably. The human bile salt pool is primarily comprised of cholic, chenodeoxycholic, and deoxycholic acids, with smaller amounts of lithocholic and ursodeoxycholic acids.[8],[9] The bile acids cholic acid and chenodeoxycholic acid are conjugated by the addition of glycine or taurine (increasing their water solubility) before they are released from the hepatocyte into the bile canaliculus. Deconjugation and dehydroxylation by gut microbial enzymes lead to the formation of the secondary bile acids deoxycholic acid (from cholic acid) and lithocholic acid (from chenodeoxycholic acid).[10] Deoxycholic and lithocholic acid are the main bile acids found in the feces of healthy individuals. The bile salt pool of animals varies considerably from that of humans, even including some bile acids that are not found in humans and that act as antagonists rather than agonists of FXR,[11] which somewhat limits our ability to rely on animal models.
Bile salts are amphipathic in nature, having both hydrophobic and hydrophilic qualities in greater or lesser amounts pending their conjugation and hydroxylation state.8 Their ability to activate different receptors also varies according to their structure. Deconjugated bile acids are more hydrophobic, and have greater detergent action, which increases their ability to facilitate solubilisation and absorption of dietary lipids and fat-soluble vitamins and to break down bacterial membranes.1,[12] Deoxycholic acid is a particularly strong antimicrobial agent, having 10 times the antimicrobial activity of cholic acid, its precursor.[13]
Herein, we look at the potential of bile acids as a nutritional therapy beyond supporting fat digestion. Much like the review on bile acids and their biological actions by de Aguiar Vallim TQ, et al. (2013), which states “We cite only a small fraction of the appropriate references, as the literature on these topics is extraordinarily extensive,”9 this article attempts to provide a brief yet concise overview of some of the research pertaining to bile acids in health and disease, and their potential as a therapeutic intervention for gastrointestinal health.
Gastrointestinal Impact of Bile Acids
The relationship between the gut microbiota and bile acids is bidirectional: bile acids affect the composition of the intestinal microbiome, and the bacteria present in the gut affect bile acid metabolism.[14] Lower levels of bile acids in the gut are associated with an overgrowth of bacteria and potential pathogens (examples being Clostridium difficile[15] and Helicobacter pylori[16]), increased inflammation, and increased bacterial translocation.[17] Bile acids have both direct and indirect antimicrobial effects: their detergent action serves to break down bacterial membranes12 and their binding with FXR induces the secretion of antimicrobial peptides.[18] The binding of bile acids with FXR also has been shown to support intestinal barrier integrity and reduce bacterial translocation.
Small Intestinal Bacterial Overgrowth and Functional Constipation
Most integrative practitioners are familiar with small intestinal bacterial overgrowth (SIBO) and the key interventions necessary for successful treatment and subsequent prevention. Most practitioners are also aware that permanent resolution of this condition is often difficult to achieve, as many patients relapse when they cease following a restrictive diet or using the antimicrobials that help keep SIBO at bay. An oft-neglected tool that may help resolve this condition and prevent its recurrence (particularly when it is constipation predominant) is the use of supplemental bile acids.
Bile acids have been shown to inhibit methanogenesis in vitro in a dose-dependent fashion, a finding supported clinically by the resolution of elevated methane production after a biliary fistula reversal surgery.[19] Bile acids have also been demonstrated to inhibit hydrogen production in a culture of faecal bacteria.[20] The feeding of cholic acid to rats, in addition to typical feed, increased cecal concentration of deoxycholic acid and, not surprisingly, significantly altered the gut microbial balance.1 In rat models of cirrhosis (a condition associated with reduced bile acid secretion[21]) and obstructive jaundice, bile acid supplementation reduced bacterial overgrowth, bacterial translocation, and the related endotoxemia,[22],[23] while in the model of cirrhosis, it also led to the normalisation of bile secretion.
As promotility agents, bile acids can help reduce the constipation that is often seen with the overgrowth of methanogenic bacteria.2,[24] Bile acids serve to hasten gut transit time by inducing fluid and electrolyte secretion as well as stimulating colonic contractions.[25] Functional constipation in approximately 15% of adults has been shown to be associated with reduced total bile acid and deoxycholic acid levels in the feces,[26] and, in a smaller percentage of children, it may also be due to altered bile acid metabolism.[27]
Clinically, supplementation with a delayed-release preparation of chenodeoxycholate at 500 or 1,000 mg/day for only four days improved bowel function and accelerated colonic transit time in women with constipation-predominate irritable bowel syndrome, with the treatment being more effective in individuals with lower bile acid synthesis rates.[28] In healthy volunteers, supplemental chenodeoxycholate has also been shown to accelerate transit time, increasing stool frequency, ease of passage, and completeness of evacuation.[29] Common adverse effects at both doses were cramping of the lower abdomen and diarrhoea. It stands to reason that side effects such as these may be eliminated at lower doses; however, these studies have not yet been done.
Inflammatory Bowel Disease
The pathogenesis of inflammatory bowel disease (IBD) may also be due in part to altered bile acid metabolism.9 Because IBD is often accompanied by dysbiosis, it shouldn’t come as a surprise that the balance of the bile acid pool is also altered in IBD. In addition to their effects on the gut microbial balance, bile acids may impact IBD through interactions with the innate and adaptive immune system, as receptors that bile acids bind (FXR and the G-protein-coupled receptor, TGR5) are expressed by cells of the immune system (including macrophages, dendritic cells, and natural killer T cells) and serve to help maintain immune tolerance.[30] Not surprisingly, this effect has contributed to the study of bile acids as a therapy for other conditions of autoimmunity and allergy.[31],[32],[33] Bile acids also stimulate hepatocyte phosphatidylcholine secretion,[34] an important component of the protective intestinal mucus barrier,[35] which has been shown to be deficient (and therapeutic when applied as a treatment) in patients with ulcerative colitis.[36],[37]
FXR and the interactions of bile acids with it affect the intestinal barrier integrity, as demonstrated by FXR knockout and bile duct ligation models.20 In patients with Crohn’s colitis, a reduced level of FXR activation in the ileum has been observed.[38] Supplementation of chenodeoxycholic acid at a dose of 15 mg/kg/day to patients in remission from Crohn’s was shown to increase ileal FXR target gene expression as well as gallbladder filling,[39] supporting the possibility that bile acid supplementation may be a means to promote the therapeutic effects associated with FXR activation. An animal model of murine colitis also supports further research in this realm, finding that treatment with an FXR agonist ameliorated inflammation, protecting the intestinal barrier and improving symptoms.[40]
Interactions of bile acids with TGR5 also have many potential mechanisms via which they impact individuals with IBD. Elimination of TGR5 expression in animals leads to abnormal colonic mucus morphology, increased intestinal permeability, and an increased susceptibility to colitis;[41] treatment with deoxycholic and lithocholic acid or a TGR5 agonist suppresses the release of pro-inflammatory mediators from lamina propria mononuclear cells isolated from inflamed tissue of Crohn’s patients;[42] and numerous other in vivo animal models show that the absence or lowered expression of TGR5 leads to increased inflammation, or that the stimulation of TGR5 with bile acids helps ameliorate it.[43],[44]
Patients with IBD have been shown to have lower levels (even more so during a flare) of the secondary bile acids—which, as mentioned, have stronger antimicrobial effects in addition to their affinity for these bile acid receptors—along with a decreased level of the bacteria in the gut that deconjugate bile acids.9,[45] For this reason, in addition to the potential that supplemental bile acids have for this condition, probiotics that deconjugate bile acids (in particular those that express bile salt hydrolase [BSH], known as BSH-active bacteria) have been suggested as a therapy. Notably, in a study of hypercholesterolemic adults, a BSH-active probiotic (Lactobacillus reuteri NCIMB 30242) not only significantly increased plasma deconjugated bile acids but also improved digestive symptoms, particularly that of diarrhoea, and reduced inflammation, as assessed by high-sensitivity C-reactive protein.[46],[47]
Although supplemental bile acids may have a role in IBD for the aforementioned reasons, they may not be appropriate for individuals with concomitant primary sclerosing cholangitis (PSC).[48] As PSC may be undiagnosed or may manifest later in the disease, monitoring of liver function tests (including alkaline phosphatase) should be considered if bile acids are used as an adjunctive therapy.
With the broad array of functions that the receptors bile acids interact with have, and the wide distribution of these receptors throughout the body, research regarding the impact of bile acids on gastrointestinal health is only a snapshot of the important role these acids play in maintaining homeostasis and health. Stay tuned for the Spring 2020 issue of FOCUS, where we look at the role bile acids play in metabolic disease, and the closely related condition of nonalcoholic fatty liver disease.
References
[1] Islam KB, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011 Nov;141(5):1773-81.
[2] Hellström PM, et al. Role of bile in regulation of gut motility. J Intern Med. 1995 Apr;237(4):395-402.
[3] Trauner M, et al. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28(1):220-4.
[4] Watanabe M, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006 Jan 26;439(7075):484-9.
[5] Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014 Aug;86:62-8.
[6] McMillin M, DeMorrow S. Effects of bile acids on neurological function and disease. FASEB J. 2016 Nov;30(11):3658-68.
[7] Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015 Nov;21(11):702-14.
[8] de Aguiar Vallim TQ, et al. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013 May 7;17(5):657-69.
[9] Baars A, et al. The Gut Microbiota as a Therapeutic Target in IBD and Metabolic Disease: A Role for the Bile Acid Receptors FXR and TGR5. Microorganisms. 2015 Oct 10;3(4):641-66.
[10] Ridlon JM, et al. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006 Feb;47(2):241-59.
[11] Sayin SI, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013 Feb 5;17(2):225-35.
[12] Begley M, et al. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005 Sep;29(4):625-51.
[13] Kurdi P, et al. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006 Mar;188(5):1979-86.
[14] Ridlon JM, et al. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014 May;30(3):332-8.
[15] Buffie CG, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015 Jan 8;517(7533):205-8.
[16] Hänninen ML. Sensitivity of Helicobacter pylori to different bile salts. Eur J Clin Microbiol Infect Dis. 1991 Jun;10(6):515-8.
[17] Slocum MM, et al. Absence of intestinal bile promotes bacterial translocation. Am Surg. 1992 May;58(5):305-10.
[18] Inagaki T, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3920-5.
[19] Florin TH, Woods HJ. Inhibition of methanogenesis by human bile. Gut. 1995 Sep;37(3):418-21.
[20] Florin TH, Jabbar IA. A possible role for bile acid in the control of methanogenesis and the accumulation of hydrogen gas in the human colon. J Gastroenterol Hepatol. 1994 Mar-Apr;9(2):112-7.
[21] Schwartz CC, et al. Bile acid metabolism in cirrhosis. V. Determination of biliary lipid secretion rates in patients with advanced cirrhosis. Gastroenterology. 1979 Dec;77(6):1177-82.
[22] Lorenzo-Zúñiga V, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003 Mar;37(3):551-7.
[23] Ding JW, et al. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993 Jan-Feb;25(1):11-9.
[24] Triantafyllou K, et al. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014 Jan;20(1):31-40.
[25] Kirwan WO, et al. Bile acids and colonic motility in the rabbit and the human. Gut. 1975 Nov;16(11):894-902.
[26] Vijayvargiya P, et al. Bile Acid Deficiency in a Subgroup of Patients With Irritable Bowel Syndrome With Constipation Based on Biomarkers in Serum and Fecal Samples. Clin Gastroenterol Hepatol. 2018 Apr;16(4):522-7.
[27] Hofmann AF, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008 Nov;47(5):598-606.
[28] Rao AS, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010 Nov;139(5):1549-58.
[29] Odunsi-Shiyanbade ST, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010 Feb;8(2):159-65.
[30] Biagioli M, Carino A. Signaling from Intestine to the Host: How Bile Acids Regulate Intestinal and Liver Immunity. Handb Exp Pharmacol. 2019;256:95-108.
[31] Ho PP, Steinman L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2016 Feb 9;113(6):1600-5.
[32] Ely PH. Is psoriasis a bowel disease? Successful treatment with bile acids and bioflavonoids suggests it is. Clinics Dermatol. 2018 May 1;36(3):376-89.
[33] Shaik FB, et al. Chenodeoxycholic acid attenuates ovalbumin-induced airway inflammation in murine model of asthma by inhibiting the T(H)2 cytokines. Biochem Biophys Res Commun. 2015 Aug 7;463(4):600-5.
[34] Hofmann AF, et al. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999 Dec 13-27;159(22):2647-58.
[35] Treede I, et al. Anti-inflammatory effects of phosphatidylcholine. J Biol Chem. 2007 Sep 14;282(37):27155-64.
[36] Stremmel W, et al. Mucosal protection by phosphatidylcholine. Dig Dis. 2012;30 Suppl 3:85-91.
[37] Stremmel W, et al. Delayed release phosphatidylcholine as new therapeutic drug for ulcerative colitis–a review of three clinical trials. Expert Opin Investig Drugs. 2010 Dec;19(12):1623-30.
[38] Nijmeijer RM, et al. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PLoS One. 2011;6(8):e23745.
[39] van Schaik FD, et al. Pharmacological activation of the bile acid nuclear farnesoid X receptor is feasible in patients with quiescent Crohn’s colitis. PLoS One. 2012;7(11):e49706.
[40] Gadaleta RM, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011 Apr;60(4):463-72.
[41] Cipriani S, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6(10):e25637.
[42] Yoneno K, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology. 2013 May;139(1):19-29.
[43] Wang YD, et al. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011 Oct;54(4):1421-32.
[44] Perino A, et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J Clin Invest. 2014 Dec;124(12):5424-36.
[45] Duboc H, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013 Apr;62(4):531-9.
[46] Jones ML, et al. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. 2012 Nov;66(11):1234-41.
[47] Jones ML, et al. Improvement of gastrointestinal health status in subjects consuming Lactobacillus reuteri NCIMB 30242 capsules: a post-hoc analysis of a randomized controlled trial. Expert Opin Biol Ther. 2013 Dec;13(12):1643-51.
[48] Zweers SJ, et al. Prolonged fibroblast growth factor 19 response in patients with primary sclerosing cholangitis after an oral chenodeoxycholic acid challenge. Hepatol Int. 2017 Jan;11(1):132-40.






1 Comment. Leave new
Very interesting info you provided regarding bile disfunction causing many problems to the digestive system and the rest of immune system need more info natural way to detect malfunctions of bile and natural remedy also does pancreas and spleen have bile ducts joining with liver bile leading to digestive system also could disfunction of biles cause blood cancer and surplus of B12 and hard stools and evacuation I prefer natural remedies once find the cause of low bile and blockage Tks.